Regulators on Friday approved two new gene therapies for sickle cell disease that doctors hope can cure the painful, inherited blood disorder that afflicts mostly Black people in the U.S.
The Food and Drug Administration said the one-time treatments can be used for patients 12 and older with severe forms of the disease. One, made by Vertex Pharmaceuticals and CRISPR Therapeutics, is the first approved therapy based on CRISPR, the gene editing tool that won its inventors the Nobel Prize in 2020. The other is made by Bluebird Bio and works differently.
“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need,” the FDA’s Dr. Nicole Verdun said in a statement announcing the approvals. “”We are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease.”
In the U.S., an estimated 100,000 people have the disease and about a fifth of them have the severe form. Sickle cell is most common among Black people and 1 in 365 Black babies are born with the disease nationally. Scientists believe being a carrier of the sickle cell trait helps protect against severe malaria, so the disease occurs more often in mosquito-prone regions such as Africa or in people whose ancestors lived in those places.
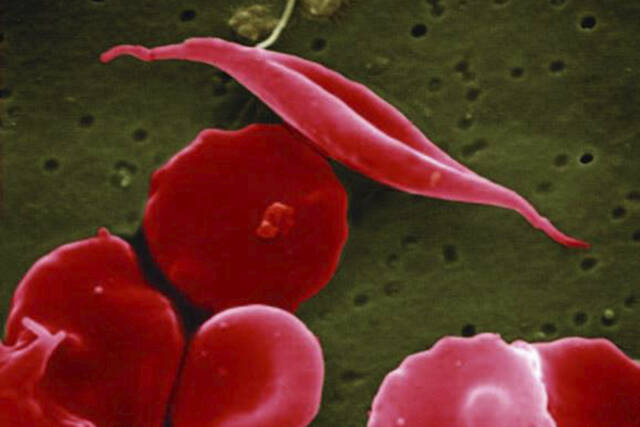
The disease affects hemoglobin, the protein in red blood cells that carries oxygen. A genetic mutation causes the cells to become sickle or crescent-shaped, which can block blood flow, causing excruciating pain, organ damage, stroke and other problems.
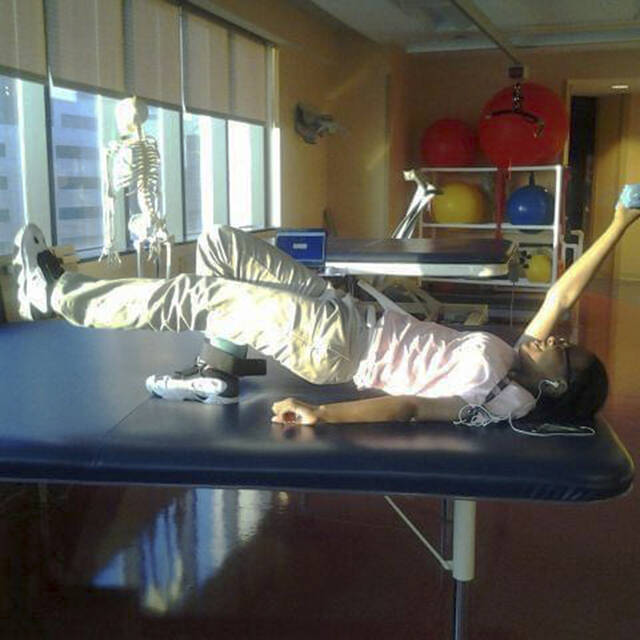
Current treatments include medications and blood transfusions. The only permanent solution is a bone marrow transplant, which must come from a closely matched donor without the disease and brings a risk of rejection.
The Vertex treatment was recently authorized in Britain and Bahrain and is under review elsewhere.