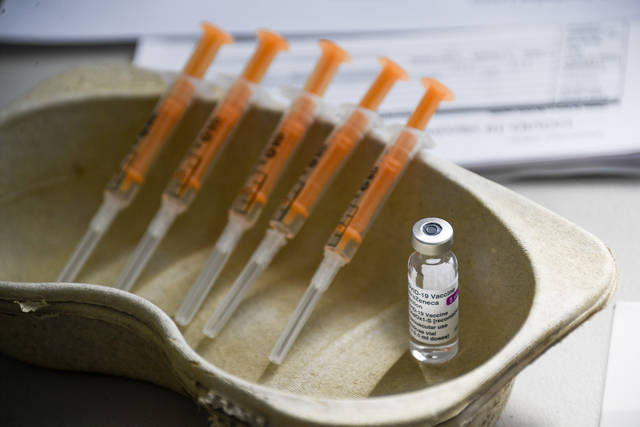
TORONTO — Canada’s National Advisory Committee on Immunization is recommending a pause on AstraZeneca covid-19 vaccinations for people under 55 for safety reasons, a person familiar with the matter told the Associated Press on Monday.
The person confirmed the recommendation on condition on anonymity as they were not authorized to speak publicly before the announcement. It remains a recommendation and it is up to each of Canada’s provinces to decide to follow it or not.
It was not immediately clear why that is being recommended but several European countries that had suspended using the vaccine over concerns it could cause blood clots have resumed administering it after the EU’s drug regulator said the vaccine was safe.
Canada is expected to receive 1.5 million does of AstraZeneca from the U.S. this week.
The vaccine is used widely in Britain, across the European continent and in other countries, but its rollout was troubled by inconsistent study reports about its effectiveness, and then more recently a scare about clots that had some countries temporarily pausing inoculations.